Discover

Rejeunesse Fine, Deep, Shape and Sparkle
All crafted specially to meet your unique needs

![]()
Treatment Areas
Explore the various treatment areas targeted by our diverse portfolio of Rejeunesse fillers.


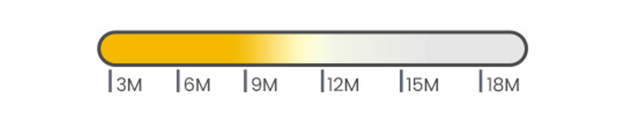
- HA 20mg/mL
- Lidocaine 0.3%
- Volume: 2.5mL
- -
- Store at 2~25ºC
- 24 months expiry


- HA 24mg/mL
- Lidocaine 0.3%
- Volume: 1.1mL
- 30G needle
- Store at 2~25ºC
- 24 months expiry


- HA 24mg/mL
- Lidocaine 0.3%
- Volume: 1.1mL
- 27G needle
- Store at 2~25ºC
- 24 months expiry


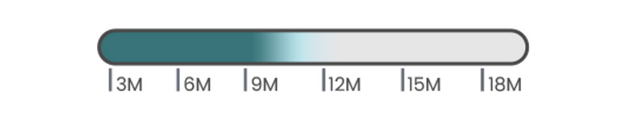
- HA 24mg/mL
- Lidocaine 0.3%
- Volume: 1.1mL
- 26G needle
- Store at 2~25ºC
- 24 months expiry
What makes Rejeunesse different?
Differentiated & Patented Technology
FHL focuses on developing regenerative cosmeceutical products using HA and polynucleotides. With a patent for long-lasting hyaluronic acid gel composition, our products maximize safety with our special DIAMOLD technology that results in below detectable levels of BDDE.

Manufacturing Process Optimised for Product Safety
Our optimized manufacturing process and clean-room environment are critical factors that result in a safe and long-duration sodium hyaluronate filler product. FHL strictly adheres to ISO13485 and cGMP guidelines governing the production of safe, stabilized, high-purity medical devices.



Raw HA
(MW: 2,200 kDa)
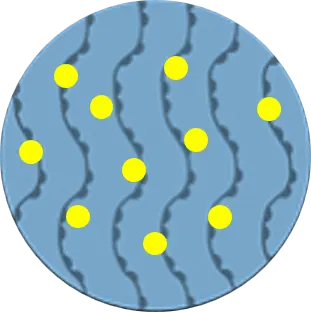
HA + Solvent Mixing
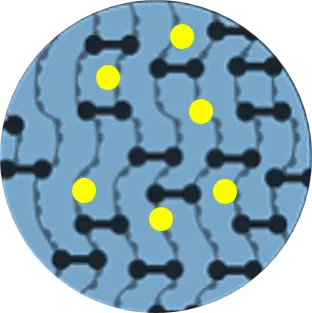
Cross-linking
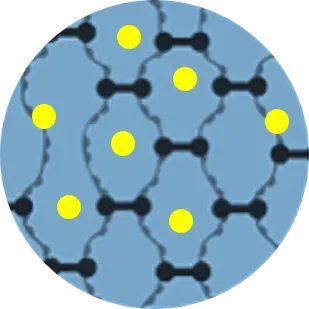
Dialysis & Molding

Homogenization (Grinding)

Filling & Sterilization
Our Quality Control Measures
All Rejeunesse products go through rigorous quality control testing and meticulous scrutiny to ensure that we deliver the highest standards of quality and safety for all your aesthetic needs.
Lidocaine concentration measurement
Endotoxin screening
Sterility testing
Injection force assessment
Heavy metal screening
and more...
pH analysis
HA identification testing
BDDE residues screening
Storage modulus testing
Our Certifications
Rejeunesse by FHL maintains exceptional quality standards, manufacturing in an internationally accredited facility that complies with ISO13485, CE, and the Korean FDA regulations and with regulatory approval in over 20 countries.